Every year, over 600,000 women are diagnosed with cervical cancer, primarily linked to human papillomavirus (HPV)1. In response, the World Health Organization (WHO) has advocated for HPV DNA testing as the preferred method for primary cervical cancer screening2. Recent trends indicate a shift away from traditional cytology tests toward this more advanced DNA-based approach.
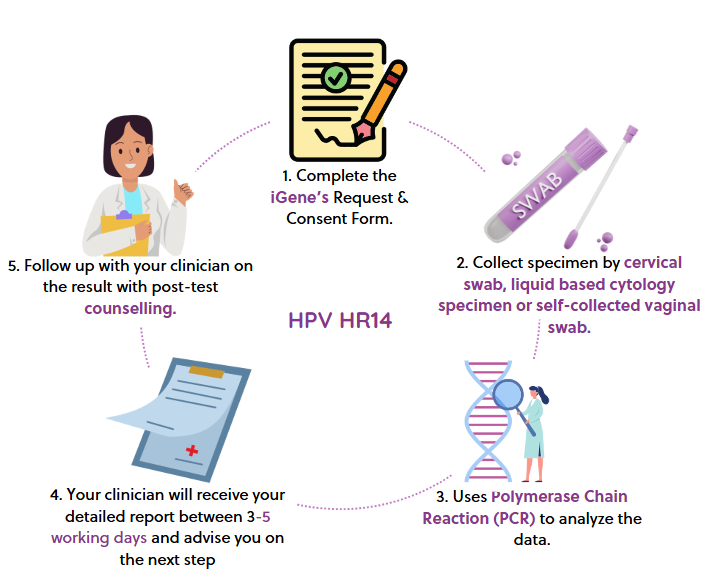
HPV HR14
A multiplex real-time Polymerase Chain Reaction (PCR) assay that enables simultaneous amplification and detection of target nucleic acids of 14 high-risk HPV types, as well as Internal Control (IC).
Test Introduction
Key Features
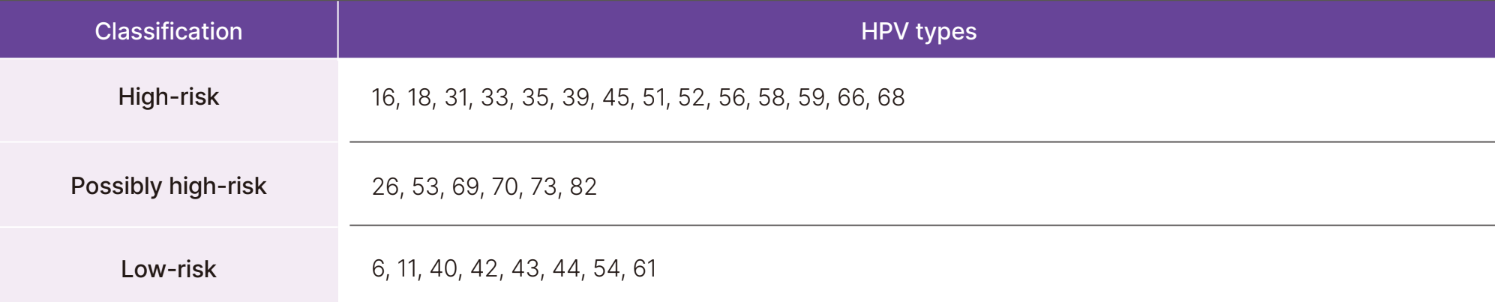
14 high-risk HPV genotypes
- Detecting and identifying 14 individual HPVs simultaneously with a single test
- Monitoring of clearance and persistance of HPV genotypes and the risk of cervical cancer
Individual Ct values
-
3 Ct PCR technology provides Ct values to infer viral load, helping clinicians to provide personalized patient care with more precise diagnosis
Self-collected samples
-
Increased accessibility of HPV screening due to alternative sample collection method, enabling early prevention of cervical cancer
Relative Sensitivity and Specificity
-
Sensitivity: 98.74%
- Specificity: 98.45%
Result Reporting
-
Provides 14 results in 1 sample (no pooling of genotypes) compared to other HPV tests in the market
HSA registered
-
Our product is now HSA-approved in Singapore, reflecting its compliance with the stringent safety and quality standards set by the Health Sciences Authority. This milestone underscores our commitment to delivering reliable and innovative healthcare solutions tailored to patient and clinical needs.
Medical treatment has to be individualised and can only be rendered after adequate assessment of your condition through appropriate clinical examination, and after discussion with your doctor. You should not rely on the information provided herein. Please note that the contents of this page are provided on the understanding that no surgical or medical advice or recommendation is being rendered.
Sources:
1) WHO guideline for screening and treatment of cervical cancer prevention. 2nd ed. Geneva: World Health Organization; 2021.
2) Latsuzbaia A et al. Effectiveness of bivalent and quadrivalent human papillomavirus vaccination in Luxembourg. Cancer Epidemiol. 2019 Dec;63:101593.
Patient Story
A Patient’s Journey: A Life-Changing Decision with early detection
Sarah, a 34-year-old lawyer in Singapore, had always prioritized her health. When her gynecologist suggested HPV HR (high-risk) testing during her annual check-up, she hesitated at first, wondering if it was necessary. However, after learning about its importance in early cervical cancer detection, she decided to proceed.
The test was quick and painless, and within a few days, Sarah received her results: they indicated a high-risk HPV type. Initially overwhelmed, her doctor explained that early detection was key and reassured her that with proper monitoring and care, cervical cancer could be prevented.
Thanks to HPV HR testing, Sarah took proactive steps, including follow-ups and lifestyle changes, to protect her health. Today, she encourages her friends and family to prioritize regular screenings, knowing that early action can make all the difference.

Other Information
Sources:
- WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, 2nd edition. Geneva: World Health Organization. 2021.
- The Global Cancer Observatory, International Agency for Research on Cancer, World Health Organization, 2021. https://gco.iarc.fr/today/data/factsheets/
cancers/23-Cervix-uteri-fact-sheet.pdf - IARC monographs on the identification of carcinogenic hazards to humans. https://monographs.iarc.who.int/list-of-classifications
- IARC Monographs on the identification of carcinogenic hazards to humans, volume 90, 2007.
- M Arbyn et al., 2020 list of human papillomavirus assays suitable for primary cervical cancer screening. Clinical Microbiology and Infection. 2021; 27:1083-1095.
- Global strategy to accelerate the elimination of cervical cancer as a public health problem. Geneva: World Health Organization. 2020.