A Revolution in Endometrial Receptivity Testing
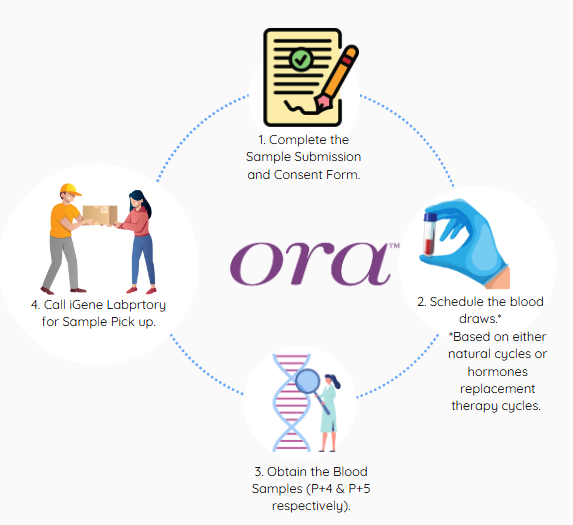
oraTM is the world’s first non-invasive endometrial receptivity test for identifying a patient’s optimal window of implantation (WOI). It assesses a combination of microRNA (miRNA) biomarkers in the bloodstream and physiological conditions to determine the status of a patient’s endometrium, providing information that can be used to optimize the timing of implantation.
This genetic test offers a non-invasive approach to identifying the ideal timing for embryo transfer, increasing the likelihood of successful implantation and pregnancy. Unlike traditional methods that involve invasive endometrial biopsies, the ORA test uses just a blood sample, giving patients a comfortable and reliable alternative.